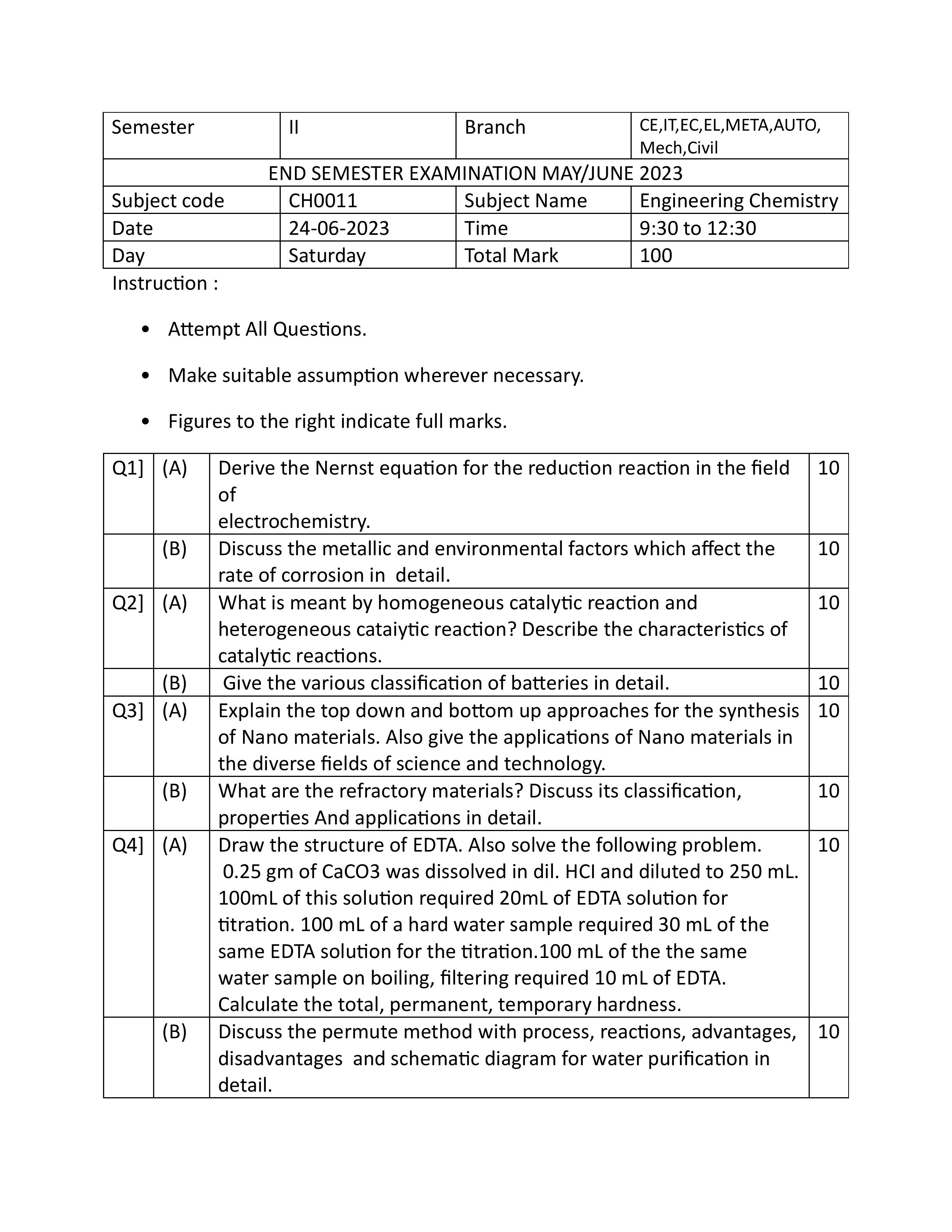
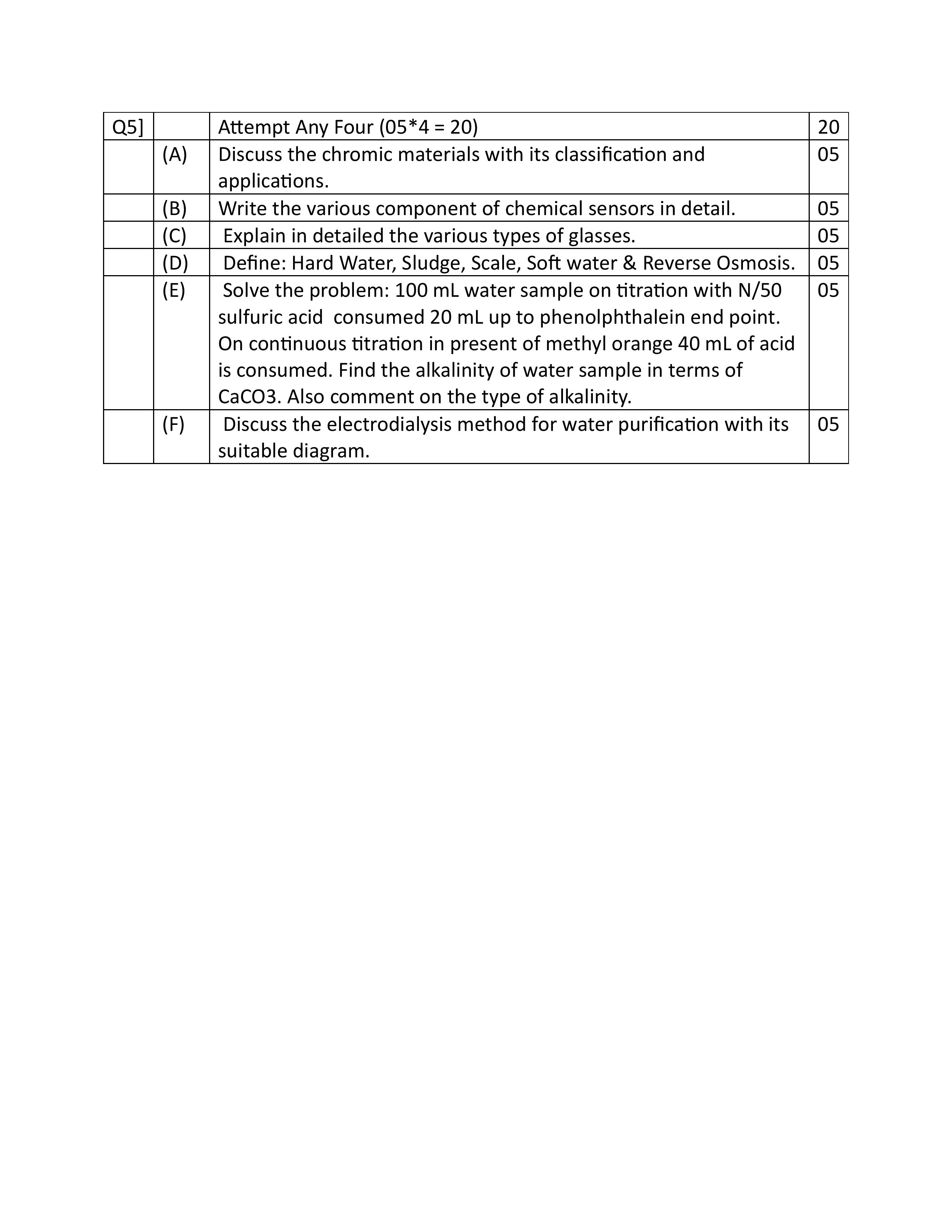
Q : 1 (A) Derive the Nernst equation for the reduction reaction in the field of electrochemistry.
ANS : For the reaction,
aA+bB=cC+dD
Where,
A & B = reactants
C & D = products
a& b = number of moles of reactants
c& d = number of moles of product
• When 1 Faraday electricity is passed through the cell, the forward reaction becomes faster.
• According to thermodynamics, the work done in the electrochemical cell is related with decrease in the energy.
-ΔG = nFΔE ………………………….1
ΔG = Free energy change
n= the number of electrons transferred
F= Faraday constant = 96500 coulombs
ΔE= cell potential in volts When the electrochemical cell is in a standard state,
When the electrochemical cell is in a standard state

Where, ΔGo = standard free energy change
ΔEo = standard cell potential
• When a reaction attains equilibrium,

• According to VontHoff’s equation,

Where,
R = gas constant (8.314 J mol-1 K-1)
K = equilibrium constant
But for electrochemical cell

Substitute this value in equation no. 3, we get:

Dividing above equation both the sides by –nF,

Q1 : (B) Discuss the metallic and environmental factors which affect the rate of corrosion in detail.
ANS :The rate and extent of corrosion depends mainly on (a) the nature of the metal (b) the
nature of the environment.
(a) Nature of the metal
- Position in the galvanic series: The extent of corrosion depends upon the position of the metal in the galvanic series. Greater the oxidation potential, the greater is the rate of corrosion. When two metals are in electrical contact, the metal higher up in the galvanic series becomes anodic and suffers corrosion. Further, the rate and severity of corrosion depend upon the difference in their positions in the galvanic series. The greater is the difference, the faster is the corrosion of anodic metal.
- Relative areas of the anode and cathode: The rate of corrosion is more when the area of the cathode is larger. When the cathodic area is larger, the demand for electrons will be more, and this results in increased rate of dissolution of metals at anodic regions.
- Purity of the metal: The impurities present in a metal creates heterogeneity and thus galvanic cells are set up with distinct anodic and cathodic areas in the metal. The higher the percentage of impurity present in a metal, the faster is the rate of corrosion of the anodic metal. For instance, impurities such as Pb and Fe in zinc lead to the formation of tiny electrochemical cells at the exposed part of the impurity and the corrosion of zinc around the impurity takes place due to local action. The effect of even traces of impurities on the rate of corrosion of zinc can be seen from the following data. It is evident that the corrosion resistance of metal may be improved by increasing its purity.
- Physical state of the metet Metal components subjected to unevenly distributed stresses are easily corroded. Even in a pure metal, the wrens under stress tend to be anodic and suffer corrosion. Caustic embrittlement takes place in stressed parts such as bends, Joints and rivets in boilers.
- Nature of the oxide film: Metals such as Mg. Ca and Ba form oxides whose is less than the volume of the metal. Hence, the oxide film formed will be porous, through which oxygen can diffuse and bring about further corrosion. On the other hand, like Al, Cr and Ni form oxides whose volume is greater than that of the metal and the non-porous oxide film so formed will protect the metal from further corrosion.
- Solubilities of the products of corrosion: Solubility of the corrosion product formed is an important factor in corrosion. If the corrosion product is soluble in the corroding medium, the corrosion of the metal will proceed faster. On the other hand, if the corrosion product is insoluble, then the protective film formed tends to suppress corrosion.
(b) Nature of the environment
- Temperature: The rate of chemical reactions and the rate of diffusion of ions increase th temperature. Hence corrosion increases with temperature. A passive metal may Some active at a higher temperature.
- Humidity: Atmospheric corrosion of iron is slow in dry air hut increases rapidly in the presence of moesture. This is due to the fact that moesture acts as the solvent for the oxygen in the air to furnish the electrolyte essential for setting up a corrosion cell. Rusting of iron increases when the relative humidit, of air reaches from 6 to 80%
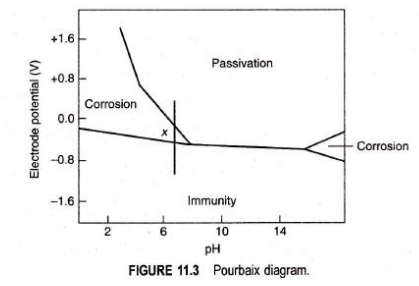
- Effect of pH: The possibility of corrosion with respect to pH of the solution and the electrode potential of the metal can be correlated with the help of a Pourhaix diagram. The Pourbaix diagram for iron in water is shown in Figure 113 The diagram shows clearly the ones of corrosion, immunity and passivity. In the diagram is a point where pill is and the electrode potentialisis present in the corrosion zone. This shows that iron rusts in water under those conditions. This is noticed to be true in actual practice also. From Figure 11.3, it is seen that the rate of corrosion can be altered by shifting the point x into immunity or passivity regions. The iron would be immune to corrosion if the potential is changed to about -0.8 V and this can be achieved by applying external current. On the other hand, the corrosion rate of iron can also be reduced by moving into the passivity region by applying positive potential. The diagram clearly indicates that the corrosion rate can also be reduced by increasing the pH of the solution by the addition of alkali without disturbing the potential.
4.Nature of the electrolyte: The nature of the electrolyte also influences the rate of corrosion. If the electrolyte consists of silicate ions, they form insoluble silicates and prevent further corrosion. On the other hand, if chloride ions are present, they destroy the protective film and the surface is exposed for further corrosion. If the conductance of electrolyte is more, the corrosion current is easily conducted and hence the rate of corrosion is increased.
5.Concentration of oxygen and formation of oxygen concentration cells: The rate of corrosion increases with increasing supply of oxygen. The region where oxygen concentration is lesser becomes anodic and suffers corrosion. Corrosion often takes place under metal washers where oxygen cannot diffuse readily. Similarly, buried pipelines and cables passing from one type of soil to another suffer corrosion due to differential aeration, e.g. lead pipeline passing through clay and then through sand. Lead pipeline passing through clay get corroded because it is less aerated than sand.
Q2 : (A) What is meant by homogeneous catalytic reaction and heterogeneous catalytic reaction? Describe the characteristics of catalytic reactions.
ANS :
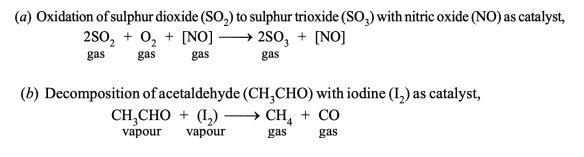
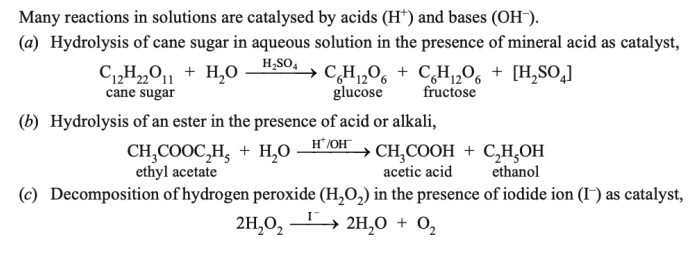
ANS : Homogeneous Catalysis
- In homogeneous catalysis, the catalyst is in the same phase as the reactants and is evenly distributed throughout. This type of catalysis can occur in gas phase or the liquid (solution) phase.
Examples of Homogeneous Catalysis in Gas Phase
Heterogeneous Catalysis
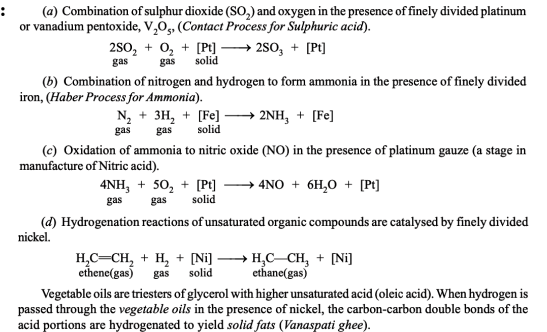
- Heterogeneous catalysis with gaseous reactants (Contact catalysis)
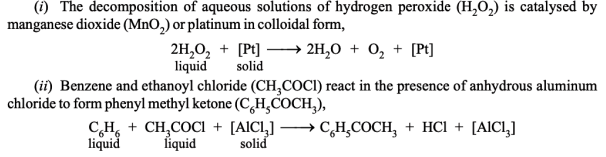
- Heterogeneous catalysis with liquid reactants
- Heterogeneous catalysis with solid reactants
- Heterogeneous catalysis with gaseous reactants (Contact catalysis)
Examples:
- Heterogeneous catalysis with liquid reactants
Examples:
- Heterogeneous catalysis with solid reactants
Example:

Q3 : (B) Give the various classification of batteries in detail.
ANS : Electrochemical cells or batteries are identified as primary (non-rechargeable) or Secondary (rechargeable), depending on their capability of being electrically recharged.
The batteries are classified as (i)primary, (ii)secondary, and (iii)reserve batteries.
(i) Primary batteries
The working principle of a primary battery is the conversion of the free energy change of the active materials during electrode processes into the electrical energy.
A battery which is not intended to be recharged and discarded when the battery has delivered all its electrical energy is known as a primary battery.
The net cell reaction of a primary battery is irreversible and as long as the active materials are present in a battery, the cell generates electrical energy. In other words, primary batteries cannot be recharged.
Example: Zn-MnO2 dry cell.
(ii) Secondary or rechargeable batteries
A secondary battery is known as a galvanic battery, which after discharge, may be restored to the fully charged state by the passage of an electric current through the cell in the opposite direction to that of the discharge.
In other words, the net cell reactions of battery can be reversed. They are storage devices for electrical energy and are known as ‘storage batteries’.
Examples: Lead-acid battery and Ni-Cd battery.
The secondary batteries have advantages over the other primary batteries that the net cell reactions can be reversed during the charging process and the current can be drawn during the discharge process.
The secondary batteries have better cycle life and capacity, so that it can be used over and over again.
The secondary batteries are classified into two types:
- Acid storage battery-lead-acid battery.
- Alkaline storage battery-Ni-Cd battery.
(iii) Reserve batteries
In these reserve types of batteries, vital component is separated from the rest of the battery prior to activation.
Under this condition, chemical deterioration or self-discharge is essentially eliminated,and the battery is capable of long-term storage.
Usually, an electrolyte is the component that is isolated.
These batteries are used, for example, to deliver high power for relatively short periods of time, in missiles, torpedoes and other weapon systems.
Example: LiV2O5 cell.
Q3 : (A)Explain the top down and bottom up approaches for the synthesis of Nano materials. Also glee the applications of Nano materials in the diverse fields of science and technology.
ANS :
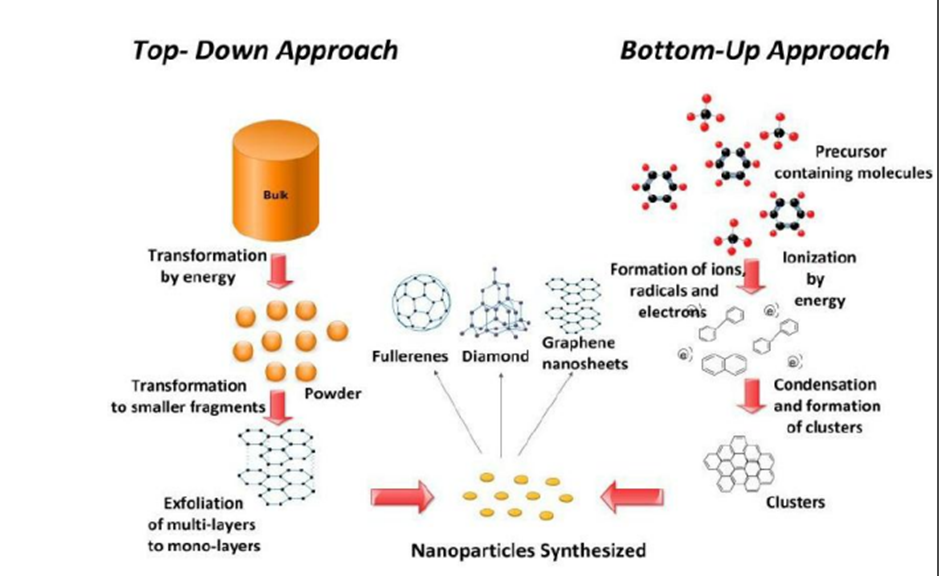
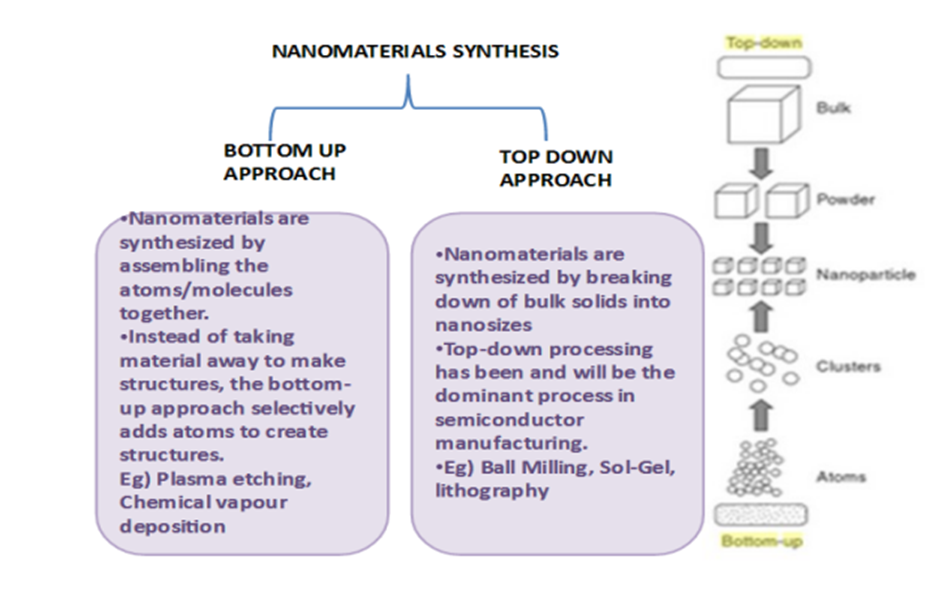
Top-down vs. bottom-up
• Top-down methods
– begin with a pattern generated on a larger scale, then reduced to nanoscale.
– By nature, aren‟t cheap and quick to manufacture
– Slow and not suitable for large scale production.
• Bottom-up methods
– start with atoms or molecules and build up to nanostructures
– Fabrication is much less expensive
Application of Nano materials:—
Nano materials are used in a variety of, manufacturing processes, products and healthcare including paints, filters, and insulation and lubricant
additives.
They are an emerging type of artificial enzyme, which have been used for wide applications in such as biosensing, bioimaging, tumor diagnosis.
In paints nanomaterials are used to improve UV protection and improve ease of cleaning.
Nanomaterials are being used in modern and human-safe insulation technologies, in the past they were found in Asbestos-based insulation
As a lubricant additive, nano materials have the ability to reduce friction in moving parts. Worn and corroded parts can also be repaired with self-
assembling anisotropic nanoparticles called TriboTEX.
Next-Generation Computer Chips : The microelectronics industry has been emphasising miniaturisation, whereby the circuits, such as transistors, resistors, and capacitors, are reduced in size. By achieving a significant reduction in their size, the microprocessors, which contain these
components, can run much faster, thereby enabling
Better Insulation Materials : Nanocrystalline materials synthesised by the sol-gel technique result in foam like structures called “aerogels.” These
aerogels are porous and extremely lightweight; yet, they can loads equivalent to 100 times their weight. Aerogels are composed of three-
dimensional, continuous networks of particles with air (or any other fluid,such as a gas) trapped at their interstices. Since they are porous and air is
trapped at the interstices, aerogels are currently being used for insulation in offices, homes, etc. By using aerogels for insulation, heating and cooling
bills are drastically reduced, thereby saving power and reducing the attendant environmental pollution.
Phosphors for High-Definition TV: The resolution of a television, or a monitor, depends greatly on the size of the pixel. These pixels are essentially
made of materials called “phosphors,” which glow when struck by a stream of electrons inside the cathode ray tube (CRT). The resolution improves with
a reduction in the size of the pixel, or the phosphors. Nanocrystalline zinc selenide, zinc sulfide, cadmium sulfide, and lead telluride synthesised by the
sol-gel techniques are candidates for improving the resolution of monitors.
Q3 : (B) What are the refractory materials? Discuss its classification, properties And applications in detail.
ANS : A refractory material or refractory is a heat-resistant material: that is, a mineral that is resistant to decomposition by heat, pressure, or chemical attack, most commonly applied to a mineral that retains strength and form at high temperatures.
Refractories are inorganic non-metallic material which can withstand high temperature without undergoing physico – chemical changes while remaining in contact with molten slag, metal and gases. They are exposed to environments above 1,000 °F
Refractory materials must be chemically and physically stable at high temperatures. Depending on the operating environment, they must be resistant to thermal shock, be chemically inert, and/or have specific ranges of thermal conductivity and of the
coefficient of thermal expansion.
It is classified as below:
1. Acidic refractories consist of acidic materials like alumina (Al2O3), and silica (SiO2).
They are impervious to acidic materials, but easily attacked by basic materials. Important
members of this group are alumina, silica, and fireclay refractories.
2. Basic refractories consist of basic materials such as CaO, MgO, etc.
These are impervious to basic materials, but easily attacked by acidic materials. Important members of this group are magnesite and dolomite refractories.
A good refractory material should have the following properties:
It should be able to withstand high temperatures generated in the furnace.
It should be able to withstand sudden alternating heating and cooling, i.e., thermal shocks.
It should be able to withstand abrasion and rough usage.
Its contraction and expansion due to the inevitable temperature variation should be minimum possible.
It should be able to withstand fluxing action of the slags and the corrosive action of gases.
It should have good heat insulating properties.
It should be chemically inactive at elevated temperatures.
It should be impermeable to gases and liquids as far as possible.
If used in electric furnaces, it must have low electrical conductivity.
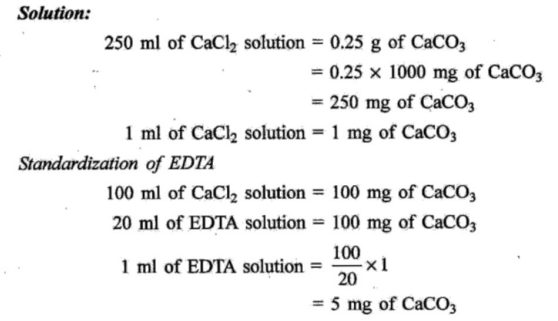
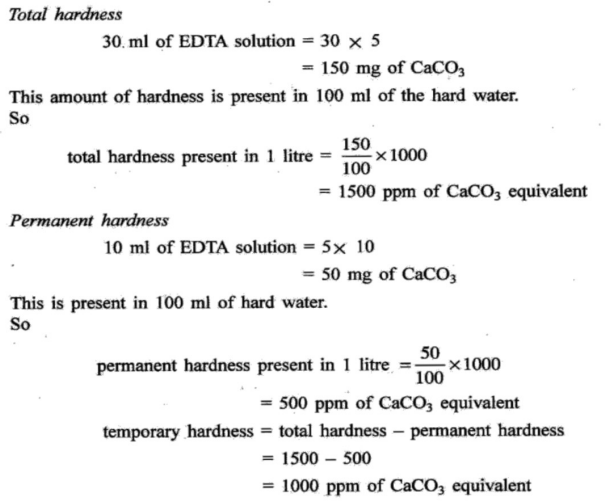
Q4 : (A) Draw the structure of EDTA. Also solve the following problem.0.25 gm of CaCO3 was dissolved in dil. HCI and diluted to 250 mL. 100mL of this solution required 20mL of EDTA solution for titration. 100 mL of a hard water sample required 30 mL of the same EDTA solution for the titration. 100 mL of the the same: water sample on boiling, filtering required 10 mL of EDTA. Calculate the total, permanent, temporary hardness.
ANS :
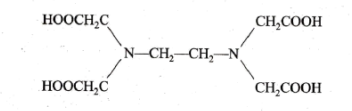
Q4 : (B) Discuss the permute method with process, reactions, advantages,disadvantages and schematic diagram for water purification in details.
ANS : ZEOLITE OR PERMUTIT PROCESS :
Zeolite – hydrated sodium alumino silicate, capable of exchanging reversibly its sodium ions for hardness- producing ions in water.
The general chemical structure of Zeolite:
Na2O.Al2O3.xSiO2.yH2O
(x = 2-10 and y = 2-6)
Two types:
(i) Natural Zeolite: non-porous.
e.g., Natrolite
(ii) Synthetic zeolite: porous & possess gel structure.
They are prepared by heating together china clay, feldspar and soda ash.
Zeolites possess higher exchange capacity per unit weight than natural zeolite.
Process:
Hard water is percolated at a specified rate through a bed of zeolite, kept in a cylinder.
The hardness-causing ions are retained by the zeolite as CaZe and MgZe; while the outgoing water contains sodium salts.

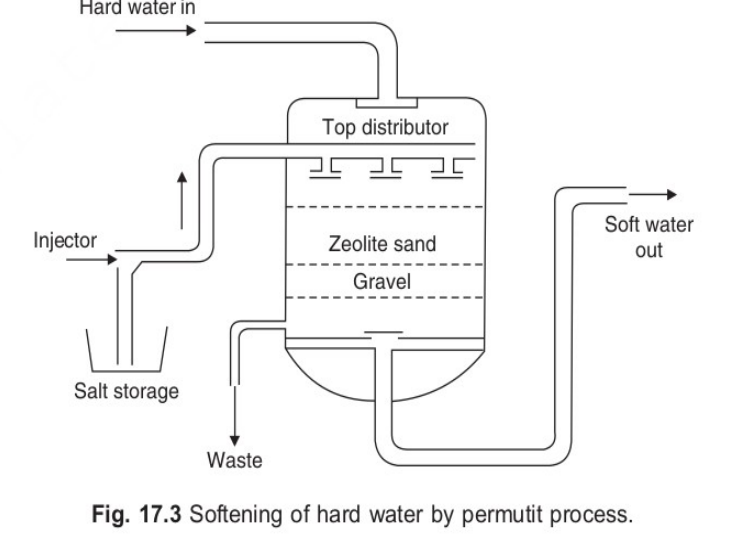
REGENERATION OF ZEOLITE:
At this stage, the supply of hard water is stopped and exhausted zeolite is
reclaimed by treating the bed with concentrated NaCl solution.
CaZe or MgZe + 2NaCl Na2Ze + CaCl2
(exhausted zeolite) (Brine) (Reclaimed zeolite) (washings)
LIMITATIONS OF ZEOLITE PROCESS:
- If the water is turbid then the turbidity causing particles clogs the pores of the Zeolite and making it inactive
- The ions such as Mn2+ and Fe2+ forms stable complex Zeolite which can not be regenerated that easily as both metal ions bind strongly and irreversibly to the zeolite structure.
- Any acid present in water (acidic water) should be neutralized with soda before admitting the water to the plant, since acid will hydrolyze SiO2
- forming silicic acid
ADVANTAGES:
● Residual hardness of water is about 10 ppm only
● Equipment is small and easy to handle
● Time required for softening of water is small
● No sludge formation and the process is clean
● Zeolite can be regenerated easily using brine solution
● Any type of hardness can be removed without any modifications to the process.
DISADVANTAGES:
● Soft water contains more sodium salts than in lime soda process
● It replaces only Ca2+and Mg2+ with Na+ but leaves all the other ions like HCO–3 and CO2 in the softened water (then it may form NaHCO3
and Na2CO3 which releases CO2 when the water is boiled and causes corrosion)
● It also causes caustic embitterment when sodium carbonate hydrolyses to give NaOH
Q5 : (A) Discuss the chromic materials with its classification and applications.
ANS :
❑ Chromic material is made by a process that induces a change in the colors of compounds. The process is called
as a Chromism.
❑ In most cases; chromism is based on a change in the electronstates of molecules.
❑ Chromic Materials are those which change their colour reversibly according to external environmental conditions, for this reason they are also called chameleon fibres
❑ Chromic materials are the general term referring to materials which radiate the colour, erase the colour or just change it because its induction caused by the external stimulus,
CLASSIFICATION OF CHROMIC MATERIALS:
1.Thermochromic material=
It is induced by heat, that is a change of temperature.
Example: Cu2HgI4 is red at 200C but black at 700C, ZnO is white at room temperature but yellow at higher temperatures.
II. Photochromic material=
It is induced by light irradiation. This phenomenon Is based on the isomerization between two different molecular structures, light induced formation of color centers in crystals, precipitation of metal particles in a glass, or other mechanisms.
Example : piropyrans,spirooxazines
III.Electrochromic material =
It is induced by the gain and loss of electrons . This phenomenon occurs in compounds with redox active sites, such as metal ions or organic radicals .
Example: methoxy biphenyls, fluorenones, benzoquinones, napthoquinones and anthraquinones
VI. Solvatechromic material =
The material is depends on the polarity of the solvent . Most solvate chromic compounds are metal complexes.
Example : piropyrans, spirooxazines
Q5 : (B) Write the various component of chemical sensors in detail.
ANS :
❑ A chemical sensor is a device that transforms chemical information (composition, presence of a particular element or ion, concentration, chemical activity, partial pressure…) into an analytically useful signal.
❑ The chemical information, mentioned above, may originate from a chemical reaction of the analyte or from a physical property of the system investigated. They can have applications in different areas such as medicine, home safety, environmental pollution and many others.
❑ Chemical sensors usually contain two basic components connected in series: a chemical (molecular) recognition system (receptor) and a physicochemical transducer. In the majority of chemical sensors, the receptor interacts analyte molecules. As a result, its physical properties are changed in such a way that the appending transducer can gain an electrical signal.
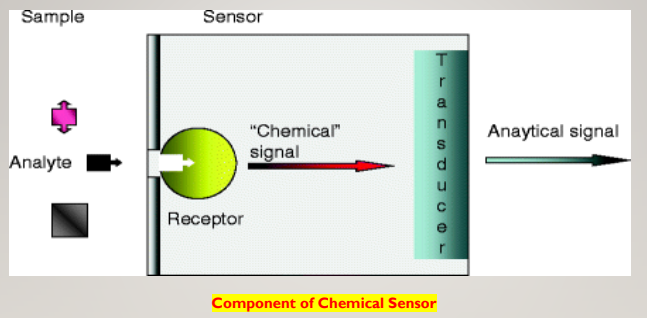
COMPONENT OF CHEMICAL SENSOR
Receptor
- The receptor is in contact with the anlyte (i.e. the sample) and its prime duty to provide high selectivity towards the desired analyte in the presence of
other chemical species . It also transforms the analyte concentration into a
chemical or physical output signal with a defined sensitivity . - The function of the receptor is fulfilled in many cases by a thin layer which is able to interact with the analyte molecules, catalyze a reaction selectively, or participate in a chemical equilibrium together with the analyte.
Transducer
- The transducer is another crucial component of the sensor.
- The transducer converts the signal generated by the receptor to a readable value .
- Every sensor should include a transducing function, i.e. the actual concentration value, a non-electric quantity must be transformed into an electric quantity, voltage, current or resistance. Some of them develop their sensor function only in combination with an additional receptor layer.
Q5 : (C) Explain in detailed the various types of glasses.
ANS :
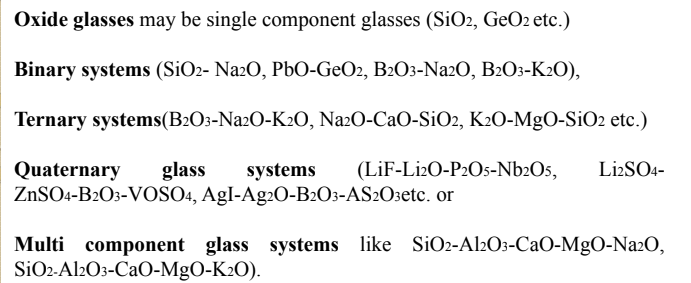
❑ Based on the composition of glasses (or sometimes based on their applications) they are classified broadly into two categories as oxide glasses and non-oxide glasses.
❑ The constituents in the glass composition play major role in the application and classification of glasses.
Non-oxide glasses are also prepared in many compositions. Some important non-oxide glasses are chalcogenide glasses.
Non-oxide glasses such as Fluorozirconate and Fluoroaluminate groups are popular for making glass fibers. These glasses are used in electrical and
optical memories.
Oxide glasses depending on composition may be further classified and some of these oxide glass systems are discussed briefly here.
Soda-Lime Glasses
🡪 The less expensive commercial glass is soda –lime glass.
🡪 These are used as window glasses because of their good light
transmission.
🡪 Soda –lime glasses are also used as glass containers, jars etc.
🡪 The chief constituents in soda-lime glass are silica (SiO2-60-75%), soda(Na2CO3-12-18%) and lime (CaCO3or MgCO3-5-12%).
🡪 Other materials are also added to these constituents to get required properties.
🡪 The main disadvantage of these glasses is they are not resistant to high temperatures and sudden thermal changes.
Borosilicate Glasses
🡪 Borosilicate glass is widely used in the manufacture of laboratory glassware, pharmaceutical containers, high-power electric bulbs etc.
🡪 Because of their good heat resistant properties and thermal shock resistance they are used in chemical industry, domestic kitchen
cooking utensils (microwave or oven ware).
🡪 The main ingredients of borosilicate glass are silica (SiO2-70-80%) and boric acid (7-13%).
🡪 Oxides of sodium, potassium and aluminum are added to borosilicate composition to get good chemical durability.
Lead Glasses
Lead glasses are widely used for decorative purposes because of their bright brilliance due to high refractive index.
Generally lead glasses contain SiO2(54-65%), PbO(18-38%),Na2O(13-15%) and other oxides in small proportions. If the PbO content is less than 18% in the glass it is called as crystal glass.
If the lead content is high (65%), the glass can be used for radiation shield glass as lead absorb rays.
Q5 : (D) Define: Hard Water, Sludge, Scale, Soft water& Reverse Osmosis.
ANS : Hard Water & Soft water:–
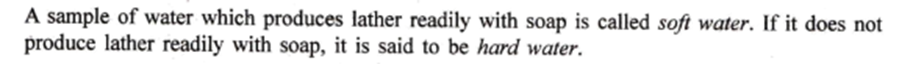
Sludge:–Sludge is a soft, loose and slimy precipitate formed within the boiler. It can be easily scrapped off with a wire brush.
Scale:–Scales are hard substances which sticks very firmly to the inner surfaces of the boiler wall.
Reverse Osmosis:–when a hydrostatic pressure in excess of osmotic pressure is applied on the concentrated side, the solvent flow is reversed from
concentrated side to dilute side, across the membrane. This principle is termed as reverse osmosis.
Q5 : (E) Solve the problem: 100 mL water sample on titration with N/50 sulfuric acid consumed 20 mL up to phenolphthalein end point. On continuous titration in present of methyl orange 40 mL of acid is consumed. Find the alkalinity of water sample in terms of CaCO3. Also comment on the type of alkalinity.
ANS :

Q5 : (F) Discuss the electrodialysis method for water purification with its suitable diagram.
ANS :
Principle:
Electrodialysis is an electrochemical process whereby electrically charged particles, ions, are transported from a raw solution (retentate, diluate) into a more concentrated solution (permeate, concentrate) through ion-selective membranes by applying an electric field.
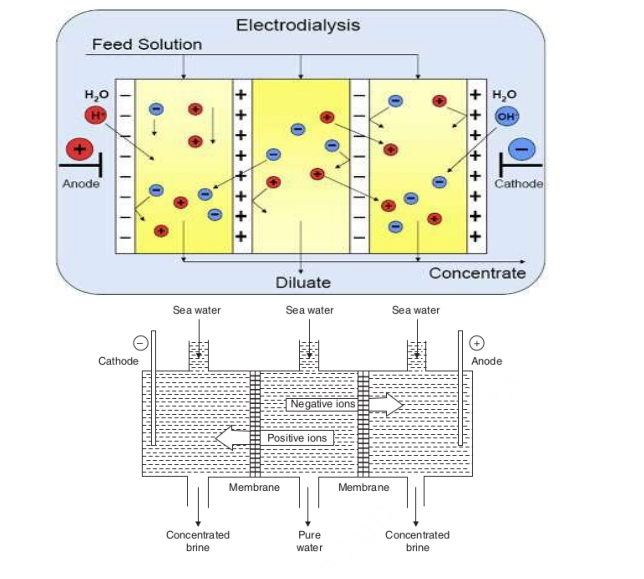
THEORY OF ELECTRODIALYSIS
● Electro dialysis chamber comprises of sheet like barriers made out of high-capacity, highly cross-linked ion exchange resins that allow passage of ions but not of water.
● There are two types : (a) Cation exchange and (b) Anion exchange membranes
● Cation exchange membranes consists of an insoluble matrix and mobile cation reside in the pore space that allows the pass through of only cations.
● Anion exchange membranes consists of an insoluble matrix and mobile anion reside in the pore space that allows the pass through of only anions.
● Cation- and Anion- exchange membranes are installed alternatively in the tank.
● By impressing electricity on the electrodes, the positive anode attracts negative ions in solution, while the negative cathode attracts positive ions in the solution.
● Na+: negative pole,
● Cl-: positive pole.
● Concentration of brine decreases : central compartment.
● It increases: two side compartment.
● Desalinated brine : removed
● Concentrated brine : replaced by fresh water.
● Electrodialysis cell : consists of a large no. of rigid plastic membrane.
➢ Saline water is passed & electric field is applied.
➢ Positive charges : repel positive ions & permit negative ion.
➢ Negative charges.
➢ Water: deprived of its salts,
➢ Salt concentration : increases in adjacent compartment.
● Advantage:
- Most compact unit,
- Economical,
- If electricity is easily available, it is best suited